Background: Brentuximab vedotin (BV) and anti-PD-1 therapies (nivolumab and pembrolizumab) have changed the therapeutic landscape for patients (pts) with classical Hodgkin lymphoma (cHL) over the past decade. Although first proven to be beneficial in relapsed and refractory cHL, these therapies have moved earlier in the treatment algorithm given superior response rates and survival compared to cytotoxic chemotherapy alone. However, there is limited data regarding outcomes in pts who progress after both BV and anti-PD-1 therapies (double refractory, DR) or become intolerant (INT) of these therapies. Knowledge of outcomes in this subset of pts is important as BV and anti-PD-1 therapies are increasingly used in front-line treatment and early relapsed cHL.
Methods: We conducted a retrospective study of adult pts from 14 U.S. academic medical centers who developed DR cHL or became INT of BV and/or anti-PD-1 therapy between January 2011 and December 2021. DR was defined as treatment failure (evidence of progression by imaging or biopsy) during therapy or within 3 months of the last dose of both BV and anti-PD-1 therapy. INT was defined as any toxicity limiting further cycles of BV or anti-PD-1 therapy. Pts with INT were either intolerant to both BV and anti-PD-1 or intolerant and refractory to BV or anti-PD-1. The primary endpoint was assessment of overall survival from time of cHL diagnosis (OS-1) and from DR or INT (OS-2). Secondary endpoints included description of pt characteristics developing DR or INT cHL, estimate of OS comparing DR versus INT, and estimate of OS stratified by prior autologous stem cell transplant (ASCT) at the time of DR/INT. Time-to-event analyses were evaluated by Kaplan-Meier methodology. INT pts were not included in time to progression (TTP) analysis for initial BV or anti-PD-1 therapy.
Results: 158 pts were eligible. Clinical characteristics at diagnosis included median age 33 years (range 16-89), 61% male, 77% white race, 66% nodular sclerosing subtype, 47% stage IV, and 56% with B symptoms. Front-line treatment was ABVD/AVD in 122 pts (77%) and BV or anti-PD-1 therapy in 28 pts (18%). 60% had primary refractory cHL (n=94). Table 1 describes pt characteristics in detail.
Initial BV treatment was after a median of 2 lines of therapy (LOT) (range 0-7). The majority (60%, n=94) were treated with BV monotherapy and 15% (n=24) were treated with BV combined with anti-PD-1 therapy. Median TTP of BV refractory pts was 168 days (95%CI 138-190). Initial anti-PD-1 therapy was after a median of 3 LOT (range 0-12). Nivolumab was the most commonly used (72%) anti-PD-1 therapy. Median TTP of anti-PD-1 refractory pts was 223 days (95%CI 166-271).
DR cHL was observed in 119 pts (75%), while 39 pts (25%) were INT. The median time from cHL diagnosis to DR/INT was 3.3 years (95%CI 0.5-23.1). At the time of DR/INT, 47% (n=75) had prior ASCT. The majority (87%, n=137) received subsequent LOTs after DR/INT (median 2, range 0-14). Table 1 describes DR/INT in detail.
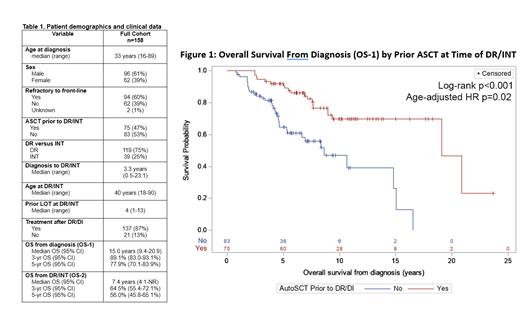
The median OS from the time of cHL diagnosis (OS-1) in all pts was 15.0 years (95%CI 9.4-20.9). There was no difference in OS-1 between DR and INT pts (log-rank p=0.83) or primary refractory and front-line relapsed pts (log-rank p=0.94). Pts who developed DR/INT at any time after ASCT had a significantly longer OS-1 compared to DR/INT without prior ASCT with a median OS of 19.1 years (95%CI 19.1-NR) versus 8.6 years (95%CI 5.3-15.0), respectively (log-rank p<0.001, Figure 1). Comparing pts with prior ASCT and no prior ASCT, the median age at DR/INT was 35 (range 20-75) versus 49 (range 18-90) years, Fisher's exact p=0.01. Adjusting for age at DR/INT did not impact the association between prior ASCT and improved OS, Cox proportional hazard ratio 0.48 (95%CI 0.25-0.90, p=0.02). Median OS from time of DR/INT (OS-2) was 7.4 years (95%CI 4.1-NR). OS-2 was not different between DR and INT pts (log-rank p=0.33).
Conclusion: To our knowledge, this is the largest cohort of pts refractory to, or intolerant of BV and anti-PD-1 therapy. OS was longer than anticipated both from diagnosis and from time of DR/INT. Pts who were DR/INT after ASCT had a significantly longer OS. Further investigation is needed to determine if OS differences by ASCT may be due to disease biology or sequencing of therapies. Importantly, OS-2 serves as a benchmark for future clinical trials evaluating DR/INT cHL pts.
Disclosures
Voorhees:Novartis: Consultancy; Recordati: Consultancy, Research Funding; AstraZeneca: Research Funding; Incyte: Research Funding; Morphosys: Research Funding. Torka:Genentech: Consultancy; Seagen: Consultancy; Genmab: Consultancy; ADC Therapeutics: Consultancy; TG Therapeutics: Consultancy; Lilly USA: Consultancy. Moyo:Kite Pharmaceuticals: Consultancy. Patel:TG Therapeutics: Consultancy, Speakers Bureau; Bristol Myers Squibb: Consultancy, Research Funding, Speakers Bureau; Trillium Therapeutics/Pfizer: Consultancy, Research Funding; Xencor: Consultancy, Research Funding; Merck: Consultancy, Research Funding; CRISPR Therapeutics: Research Funding; Sunesis Pharmaceuticals: Research Funding; Morphosys: Consultancy; Nurix: Research Funding; Pharmacyclics/Janssen: Consultancy, Research Funding; Kite: Consultancy, Research Funding, Speakers Bureau; AstraZeneca: Consultancy, Research Funding, Speakers Bureau; BeiGene: Consultancy; Fate Therapeutics: Research Funding; Epizyme: Consultancy, Research Funding; Curis, Inc: Research Funding; Genentech/Roche: Consultancy, Research Funding; Caribou Biosciences: Consultancy; Loxo Oncology: Consultancy, Research Funding; MEI Pharma: Consultancy, Research Funding; ADC Therapeutics: Consultancy; Adaptive Biotechnologies: Research Funding; Abbvie: Consultancy. Saeed:Morphosis: Honoraria; Novarits: Consultancy; Epizyme: Consultancy. Svoboda:Incyte: Consultancy, Research Funding; Merck: Research Funding; BMS: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; Genmab: Consultancy; SEAGEN: Consultancy, Research Funding; Adaptive: Consultancy, Research Funding; Astra Zeneca: Consultancy, Research Funding; ADCT: Consultancy; TG Therapeutics: Research Funding; Atara: Consultancy. Desai:Seagen: Honoraria. Grover:Tessa Therapeutics: Research Funding; Caribou Biosciences: Honoraria; Seagen: Honoraria; Genentech: Honoraria; Kite: Honoraria; ADC Therapeutics: Consultancy, Honoraria; Novartis: Honoraria; Sangamo: Current holder of stock options in a privately-held company; Seattle Genetics: Consultancy. Epperla:Incyte: Speakers Bureau; ADC Therapeutics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Merck: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Beigene: Research Funding, Speakers Bureau; Lilly: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees.